Translate this page into:
Perinuclear antineutrophil cytoplasmic antibody-associated pulmonary vasculitis masquerading as lung metastasis
Corresponding Author:
Surya Kant
Department of Respiratory Medicine, King George’s Medical University, Lucknow, Uttar Pradesh
India
skantpulmed@gmail.com
| How to cite this article: Bajpai J, Kant S, Verma AK, Bajaj DK. Perinuclear antineutrophil cytoplasmic antibody-associated pulmonary vasculitis masquerading as lung metastasis. Natl Med J India 2018;31:343-344 |
Abstract
Churg-Strauss syndrome (CSS) is an uncommon systemic disorder leading to inflammation of small and medium vessels by an immunological mechanism. Lungs are the most frequent organ affected. It is an allergic granulomatosis with polyangiitis associated with the presence of perinuclear antineutrophil cytoplasmic antibody. We describe a rare presentation of CSS as bilateral multiple cavitating nodules in a 50-year-old man with diabetes.
Introduction
Pulmonary vasculitis is usually a manifestation of a systemic disorder leading to inflammation of vessels of different sizes by a variety of immunological mechanisms.[1] Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis is characterized by pauci-immune necrotizing vasculitis of the small blood vessels, which comprise 3 different clinical syndromes such as eosinophilic granulomatosis with polyangiitis (EGPA, previously called Churg–Strauss syndrome [CSS]), granulomatosis with polyangiitis (previously called Wegener granulomatosis) and microscopic polyangiitis.[2] A lung lesion is a common and important clinical feature in ANCA-associated vasculitis (AAV). The incidence of AAV is 20 per million population.[3] Of the 3 pauci-immune vasculitis entities, CSS is the least common. Its prevalence is 1–3 per million population.[4] Although the pathogenesis of CSS remains poorly understood, ANCA is believed to play a role in the pathogenesis of CSS.[5]
The Case
A 50-year-old ex-smoker with diabetes, hypertension and bronchial asthma presented with complaints of progressive dyspnoea for 5 months, cough with expectoration, weight loss and night sweats for 3 months. He also complained of fever for the past 2 months, which was intermittent and low grade, and not associated with chills and rigors. He had no history of haemoptysis, haematuria, skin rash and joint pain. He had not taken antitubercular therapy in the past. There was no family history of diabetes, hypertension, malignancy or tuberculosis. He had suffered from bronchial asthma for the past 20 years. He was on a combination of formoterol and budesonide inhalation therapy. There was no history of palpable purpura or peripheral neuropathy.
The white blood cell count was 11 000/cmm, differential leucocyte count: polymorph 60%, lymphocyte 25%, eosinophil 13%, monocyte 1% and basophil 1%. Haemoglobin level was 9.6 g/dl. His chest X-ray posteroanterior (PA) view [Figure - 1] showed multiple bilateral excavating nodules of variable sizes. Sputum smear for acid-fast bacilli and Gram stain were negative. His contrast-enhanced CT of the thorax [Figure - 2] showed multiple, small, rounded, discrete and confluent lesions of varying sizes randomly scattered in both lungs with predominant involvement of bilateral lower lobes, few of the lesions had irregular and spiculated outer margins. Multiple small (<1 cm) lymph nodes were seen in the pre- and paratracheal, precarinal and subcarinal regions. The erythrocyte sedimentation rate (ESR) was raised 110 mm, serum C-reactive protein was 111.9 mg/dl, urinary protein spot was 23 mg/dl, urinary creatinine spot 30.2 mg/dl and CT-guided fine-needle aspiration cytology (FNAC) and biopsy were done from the right lung nodule. While FNAC was inconclusive, definitive biopsy was suggestive of inflammatory infiltrate comprising lymphocytes, plasma cells and neutrophils with few micro-abscesses along with areas of fibrosis. There was no evidence of granuloma or malignancy. Positron emission tomography scan [Figure - 3] showed multiple scattered mildly metabolically active parenchymal nodular lesions with a few of them having cavitation. Directly quantitative enzyme immune assay test was done to detect autoantibodies against myeloper-oxidase (MPO) antigen. Anti-MPO antibodies in the serum were 27.59 U/ml in and perinuclear ANCAs (p-ANCAs) were positive. Spirometry showed bronchial obstruction with reversibility and evaluation of the ear, nose and throat revealed paranasal sinusitis. Asthma and hypereosinophilia are prime components of CSS in most cases. A positive p-ANCA test, peripheral eosinophilia and history suggestive of bronchial asthma, sinusitis favour CSS and with these findings we started steroid and cyclophosphamide pulse therapy. The patient’s symptoms improved after 3 cycles. Chest X-ray PA view [Figure - 4] after therapy was suggestive of clearing of the nodules.
 |
| Figure 1: Cross-lagged analysis results. Note: *p<0.05; †p<0.01, ‡p<0.001 |
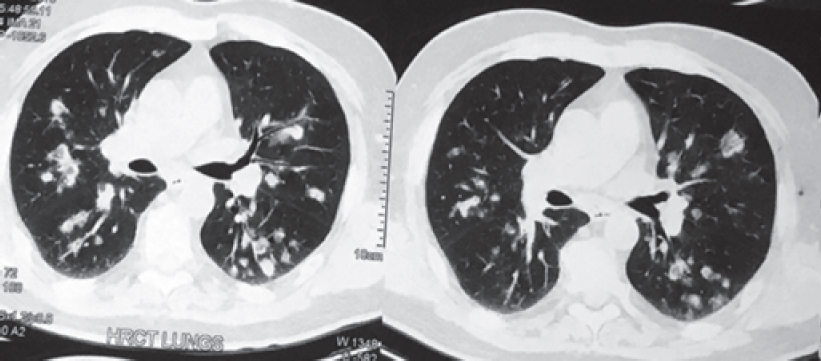 |
| Figure 2: Contrast-enhanced computed tomography thorax lung window showing bilateral diffuse cavitating nodule in middle and lower zone |
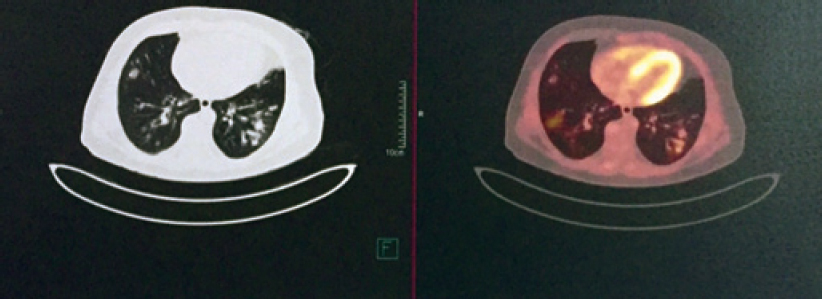 |
| Figure 3: Positron emission tomography scan showing mild uptake in pulmonary nodule |
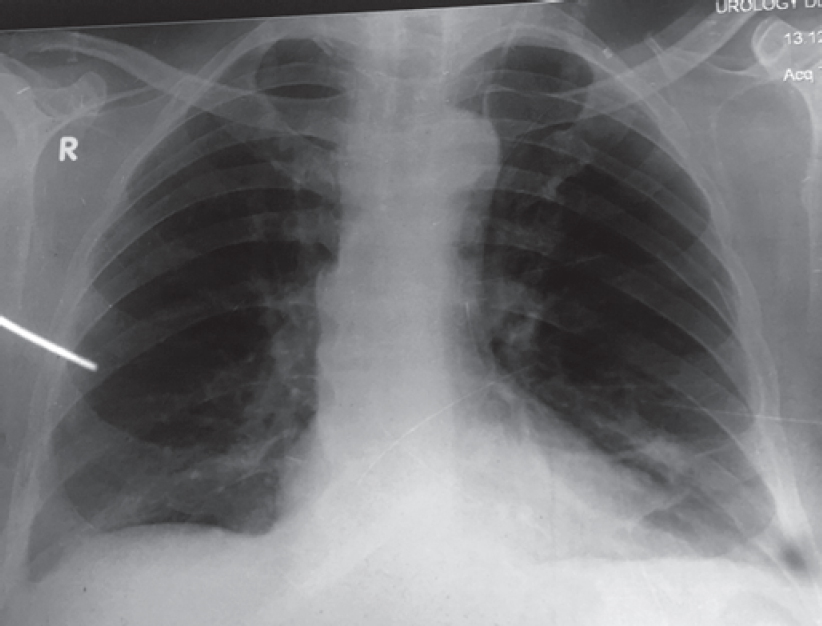 |
| Figure 4: Chest X-ray posteroanterior view showing complete resolution of pulmonary nodule after cyclophosphamide pulse therap |
Discussion
Churg-Strauss syndrome (also known as EGPA) was first described in 1951 by Churg and Strauss. It is an uncommon small vessel vasculitis with multiorgan involvement. The typical presentation of CSS is a triad of asthma, sinusitis and eosinophilia. The most commonly involved organ is the lung followed by the skin, peripheral nervous system, heart, kidney and gastrointestinal tract. Involvement of the skin in the form of purpura and nodules, and neuropathy are common in CSS. However, these clinical findings were not present in our patient. Multiple diffuse bilateral cavitating nodules are present in <10% of patients with CSS.[6] The most common radiological finding is a pattern of ground glass opacities and consolidations in a patient with a history of asthma and eosinophilia. Such a syndrome presents as a spectrum of histological manifestations in the lungs such as asthmatic bronchitis, eosinophilic pneumonia, extravascular granulomas and necrotizing vasculitis. CSS is a form of necrotizing vasculitis in several organs, with eosinophilic tissue inflammation and extravascular granulomas being present in asthmatics with fever and peripheral hypereosinophilia.[7] Nodular lesions are less common findings and they cavitate rarely.[8] The diagnosis of CSS is based on clinical features with laboratory and/or histological findings. There is no pathognomonic laboratory test for the diagnosis of CSS. The majority of patients with CSS have eosinophilia, high C-reactive protein and high serum IgE levels. About 40%–60% of CSS patients have positive p-ANCA.[9] There are 2 laboratory methods to diagnose ANCA—indirect immunofluorescence (IIF) test and antigen-specific ELISA method. The diagnostic yield varies based on the clinical probability and the type of assay used. It is common practice to use IIF as a screening tool for ANCA and antigen-specific tests for proteinase 3 and MPO as a definitive test in the second step. The pitfall with IIF is the lack of information regarding the specific antigenic target(s) of ANCA.[10],[11]
ANCA is also seen in some malignancies such as hepatocellular carcinoma and haematological malignancy but is not diagnostic.
Clinically, our patient had bronchial asthma and sinusitis and laboratory tests were suggestive of hypereosinophilia, positive p-ANCA antibodies and CECT thorax showed bilateral diffuse nodules. On the basis of these findings, we made a diagnosis of CSS and started cyclophosphamide pulse therapy along with steroids. After 3 cycles, the patient’s symptoms improved as did the radiological findings. Despite the fact that CECT thorax was in favour of a metastatic nodule, his clinical and immunological pattern supported a diagnosis of EGPA.
Acknowledgement
Dr A.K. Pradhan, Assistant Professor, Department of Cardiology, King George’s Medical University, Lucknow, Uttar Pradesh, India, for his constant support and motivation.
Conflicts of interest. None declared
| 1. | Castañer E, Alguersuari A, Gallardo X, Andreu M, Pallardó Y, Mata JM, et al. When to suspect pulmonary vasculitis: Radiologic and clinical clues. Radiographics 2010;30:33-53. [Google Scholar] |
| 2. | Pagnoux C, Guilpain P, Guillevin L. Churg-Strauss syndrome. Curr Opin Rheumatol 2007;19:25-32. [Google Scholar] |
| 3. | Conron M, Beynon HL. Churg-Strauss syndrome. Thorax 2000;55:870-7. [Google Scholar] |
| 4. | Zwerina J, Eger G, Englbrecht M, Manger B, Schett G. Churg-Strauss syndrome in childhood: A systematic literature review and clinical comparison with adult patients. Semin Arthritis Rheum 2009;39:108-15. [Google Scholar] |
| 5. | Vaglio A, Martorana D, Maggiore U, Grasselli C, Zanetti A, Pesci A, et al. HLA- DRB4 as a genetic risk factor for Churg-Strauss syndrome. Arthritis Rheum 2007;56:3159-66. [Google Scholar] |
| 6. | Hansell DM. Small-vessel diseases of the lung: CT-pathologic correlates. Radiology 2002;225:639-53. [Google Scholar] |
| 7. | Keogh KA, Specks U. Churg-Strauss syndrome. Semin Respir Crit Care Med 2006;27:148-57. [Google Scholar] |
| 8. | Baldini C, Talarico R, Della Rossa A, Bombardieri S. Clinical manifestations and treatment of Churg-Strauss syndrome. Rheum Dis Clin North Am 2010;36:527-43. [Google Scholar] |
| 9. | Abril A. Churg-Strauss syndrome: An update. Curr Rheumatol Rep 2011;13: 489-95. [Google Scholar] |
| 10. | Vassilopoulos D, Hoffman GS. Clinical utility oftesting for antineutrophil cytoplasmic antibodies. Clin Diagn Lab Immunol 1999;6:645-51. [Google Scholar] |
| 11. | Csernok E, Moosig F. Current and emerging techniques for ANCA detection in vasculitis. Nat Rev Rheumatol 2014;10:494-501. [Google Scholar] |
Fulltext Views
1,217
PDF downloads
262




