Translate this page into:
Frontline use of bevacizumab in ovarian cancer: Experience from India
2 Department of Medical Oncology, Dr B.R. Ambedkar Institute Rotary Cancer Hospital, New Delhi 110029, India
Corresponding Author:
Richa Vatsa
c/o Vikash Kumar, Type V, Quarter No. 02, AG Colony, Shastri Nagar, Thatipur, Gwalior 474011, Madhya Pradesh
India
dr.richavatsa@gmail.com
| How to cite this article: Vatsa R, Kumar L, Kumar S, Roy KK, Singh N, Meena J. Frontline use of bevacizumab in ovarian cancer: Experience from India. Natl Med J India 2018;31:15-18 |
Abstract
Background Ovarian cancer is the second most common gynaecological malignancy in India. Despite relatively high response rates to first-line carboplatin and paclitaxel-based chemotherapy in epithelial ovarian cancer (EOC), the majority of patients experience multiple relapses and finally become resistant. Vascular endothelial growth factor (VEGF) promotes progression of ovarian cancer. Bevacizumab, a recombinant humanized monoclonal antibody directed against VEGF-A is an anti-angiogenesis agent. Data on the use of bevacizumab for EOC from India are not available. We, therefore, studied the use of bevacizumab in ovarian cancer.Methods In this prospective, non-randomized study, 10 patients who received bevacizumab were compared with 20 age- and stage-matched controls. After maximal surgical debulking, patients in the bevacizumab arm received bevacizumab 15 mg/kg i.v. on day 1 every 3 weeks followed by paclitaxel and carboplatin from cycle 1. After 6 cycles, bevacizumab was continued for 1 year. Controls received paclitaxel 1 75 mg/m2 and carboplatin only for 4–8 cycles. The outcome measures were adverse effects and progression-free survival.
Results Haematological toxicity (i.e. neutropenia, thrombocytopenia and anaemia) was similar in both arms. Hypertension (40% v. 10%, p = 0.04) and bleeding-related complications (50% v. 0%, p = 0.002) were more in the bevacizumab arm. However, gastrointestinal (GI) perforations were not increased. The median progression-free survival was similar in both arms; 26 months versus 21 months (p = 0.57).
Conclusion In this small group of patients, addition of bevacizumab increased the toxicity of chemotherapy.
Introduction
Ovarian cancer is the second most common gynaecological malignancy in India.[1] The overall 5-year survival rates are 90% for early-stage disease (The International Federation of Gynaecology and Obstetrics [FIGO] stages IA and IB) and 27% for advanced-stage epithelial ovarian cancer (FIGO stages III and IV).[2] Despite relatively high response rates to first-line carboplatin and paclitaxel-based chemotherapy, the majority of patients experience multiple relapses during the course of disease and finally become resistant to the chemotherapy.
Vascular endothelial growth factor (VEGF) promotes progression of ovarian cancer[3] and patients with epithelial ovarian cancer (EOC) with high levels of VEGF have a poor outcome.[4] Bevacizumab, a recombinant humanized monoclonal antibody, directed against VEGF-A is an anti-angiogenesis agent. This novel agent has shown efficacy both as first-line,[5],[6],[7],[8] and second-line treatment for refractory and recurrent EOC.[9],[10],[11],[12]
There are little data on the use of bevacizumab for EOC in India. We assessed the use of bevacizumab therapy in addition to standard adjuvant chemotherapy with paclitaxel and carboplatin in patients with EOC.
Methods
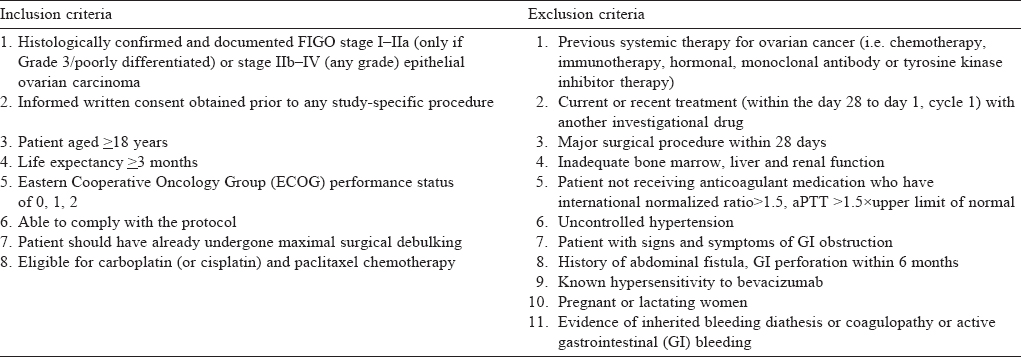
The prospective, non-randomized study was done in the Departments of Obstetrics and Gynaecology, and Medical Oncology, All India Institute of Medical Sciences, New Delhi from May 2011 to November 2015. Patients received bevacizumab in addition to standard adjuvant chemotherapy (n=10; cases) or only standard adjuvant therapy (n=20; controls). The controls were matched for age, stage and grade with the cases. [Table - 1] lists the inclusion and exclusion criteria. All patients underwent maximal surgical debulking before chemotherapy and none of the patients were lost to follow-up.
The cases received bevacizumab 15 mg/kg i.v. on day 1 every 3 weeks followed by paclitaxel and carboplatin from cycle 1. After 6 cycles, they continued to receive bevacizumab 15 mg/kg i.v. infusion every 3 weeks for 1 year. Bevacizumab was started 6 weeks after surgery.
The controls received paclitaxel 175 mg/m2 and carboplatin (AUC 6) on day 1 every 3 weeks from cycle 1. They received a minimum of 4 and maximum of 8 three-weekly post-surgical chemotherapy cycles until disease progression or the occurrence of unacceptable toxicity.
The primary outcome was to assess the safety profile of bevacizumab when added to carboplatin and paclitaxel chemotherapy as frontline treatment of EOC and the secondary aim was to assess the efficacy of bevacizumab as measured by progression-free survival (PFS). Toxicity was graded according to common terminology criteria for adverse events (CTCAE) version 4.0, provided by the National Institutes of Health and National Cancer Institute.
Patients were examined for toxicity before every cycle by clinical and laboratory parameters. Clinical parameters included assessment for nasal bleeding, gastrointestinal (GI) bleeding, haematuria, symptoms of GI perforation and measurement of blood pressure. Blood pressure was assessed before the start of each chemotherapy cycle, after 30 minutes of starting and at the end of bevacizumab infusion. Before each cycle of chemotherapy, investigations done for toxicity assessment were: complete blood count, liver function test, prothrombin time, activated partial thromboplastin time (aPTT) and urine albumin by dipstick. If the dipstick test was abnormal a 24-hour urine protein was done. These tests were done in all patients before each cycle of chemotherapy and every 3 months after completion of 6 cycles of chemotherapy.
Disease progression was assessed by the appearance of new lesions radiologically or clinically, or CA-125 criteria of disease progression. Radiologically disease progression was assessed by the response evaluation criteria in solid tumours (RECIST).
Computed tomography for disease progression was done at the end of cycles 3 and 6, and then every 6 months. Follow-up for PFS was done till 30 days after the last treatment cycle with bevacizumab.
Ethical clearance was obtained, and informed written consent was taken from all the patients.
Statistical analysis
Descriptive statistics are presented as mean, standard deviation, and median and range. Frequency distribution was compared using the Pearson chi-square and Fisher’s exact test as appropriate. PFS was defined as the time from the date of allotment of the treatment to the date of death from any cause or evidence of disease progression, whichever occurred first. Disease progression was defined according to the RECIST criteria.[13] All the statistical analyses were done using statistical package SPSS-IBM version 19.0. p<0.05 was considered statistically significant.
Results
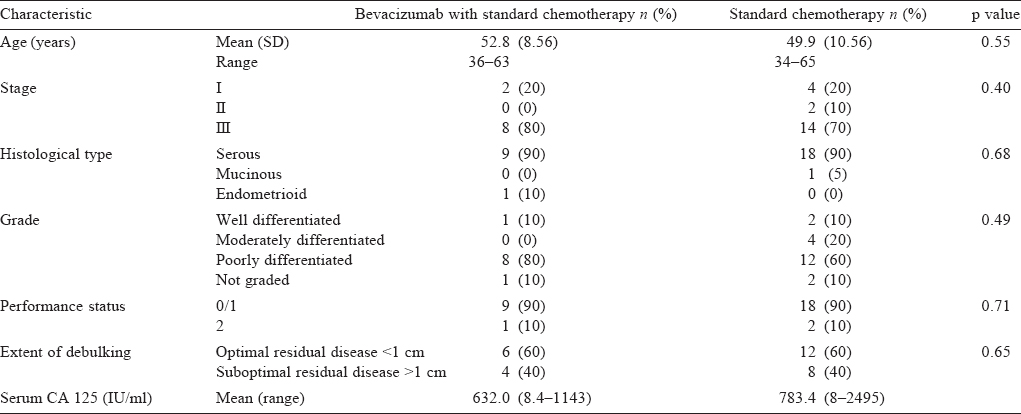
The characteristics of the patients receiving bevacizumab or standard adjuvant therapy were similar [Table - 2] and the factors that could influence treatment outcome were also equally distributed among the two groups.
While neutropenia, thrombocytopenia and anaemia were similar in the cases and controls, proteinuria, bleeding complications, hypertension and GI perforation were more frequent in the bevacizumab group. This difference was significant in the bleeding-related complications (mainly grade 1 mucocutaneous bleeding) and hypertension of grade 2 or more [Table - 3]. The other adverse events related to bevacizumab, including GI perforation or fistula, and proteinuria of grade 3 and more were not significantly different in the two groups.
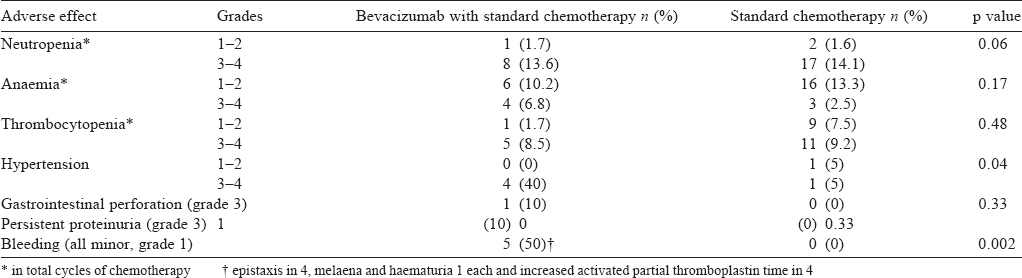
Of the 10 cases, 4 developed hypertension, which was grade 3 and 2 of the 20 controls developed hypertension, one had grade 2 and another grade 3. The difference was statistically significant (p=0.04).
All the bleeding episodes were grade 1. Three patients had grade 1 epistaxis, one patient had grade 1 haematuria, and one patient had grade 1 epistaxis and melaena both at the same time. All these episodes resolved on expectant management. Two of the 10 patients had increased aPTT, one was asymptomatic and another was symptomatic with grade 1 epistaxis that resolved on expectant management. None of the patients in the standard therapy group developed bleeding-related complications. The difference between two groups was statistically significant (p=0.002).
The median PFS was similar in both groups; 26 months for the bevacizumab group and 21 months for the standard therapy group (p=0.57; [Figure - 1]).
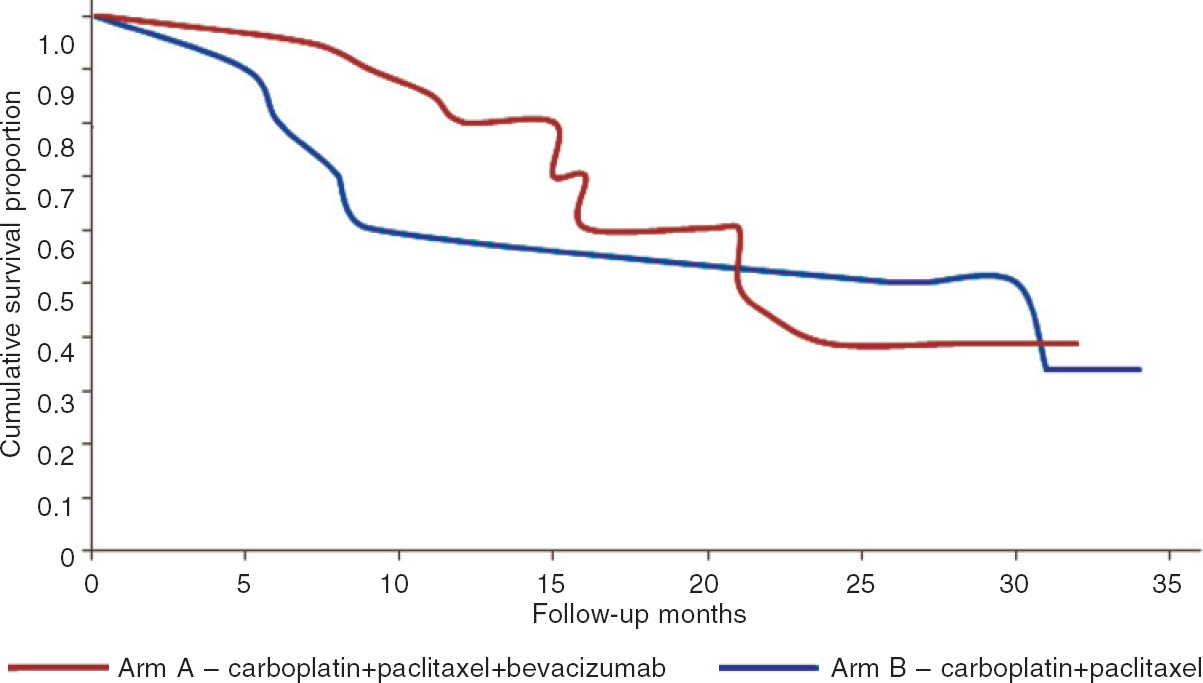 |
| Figure 1: Comparison of progression-free survival in bevacizumab with standard chemotherapy, and the standard chemotherapy groups |
In the bevacizumab group, 4 of 10 patients discontinued treatment prematurely, 2 because of disease progression after PFS of 9 and 5 months each; 1 because of development of a jejunal perforation and disease progression both after PFS of 6 months; and 1 (10%) because of development of adverse effect of persistent proteinuria of grade 3 (24 hour urinary protein of 2.5 g/day) after PFS of 8 months. All patients in the standard therapy group completed 6 cycles of chemotherapy.
Discussion
Our study shows that patients who received bevacizumab had the same frequency of haematological toxicities (anaemia, thrombocytopenia and neutropenia) as the standard therapy group. Various side-effects due to the anti-VEGF effect of bevacizumab such as hypertension (40% v. 5%), bleeding-related complications (50% v. 0%) and proteinuria (10% v. 0%) were more in the bevacizumab than in the standard therapy group. All the side-effects were manageable except proteinuria in one patient where the drug had to be discontinued. Similarly Micha et al,[5] reported high rates of hypertension (45% grades 1–2 and 10% grades 3–4). These side-effects were also seen in a phase 2 trial by Burger et al.,[6] hypertension, grade 1 in 12.9% and grade 3 in 9.7%; proteinuria, grades 1–2 in 30.6%; and haemorrhage, grade 1 in 22.6%. A similar higher incidence of hypertension and haemorrhagic complications in patients treated with bevacizumab was seen in other larger phase 3 trials too.[6],[7] GI perforation (jejunal) occurred in 1 patient after the fifth cycle of chemotherapy, and the patient needed a resection and anastomosis of the small intestine. No further bevacizumab was given to the patient. Around 2.4% of patients with solid tumours develop GI perforation on bevacizumab treatment.[14] In the ICON7 study,[7] GI perforation was seen in 1.3% and 0.4% of patients in the bevacizumab and control groups, respectively. In the GOG 218,[6] GI perforation was seen in 2.6%, 2.8% and 1.2% in arm III (chemotherapy+ bevacizumab followed by continuation of bevacizumab), II (chemotherapy+bevacizumab) and I (chemotherapy only), respectively.
Bleeding complications were also higher in the ICON7[7] study; occurred in 39.6% of those who received bevacizumab compared to only 11.6% in those who did not. In GOG 218[6] it was seen in 2.1%, 1.3% and 0.8% of arms III, II and I, respectively. The higher bleeding-related complications may be secondary to tumour necrosis and decreased renewal capacity of the endothelial cells.[15]
No arterial or venous thromboembolism or intracranial bleeding, known complications of bevacizumab therapy, were seen in any patient. There was no mortality due to treatment with bevacizumab treatment. Two large phase 3 trials that used bevacizumab as first-line treatment of EOC (GOG 218[6] and ICON7[7]) had modest but statistically significant gains in PFS. A phase 3 trial by Lauraine et al.[11] in patients with recurrent EOC showed a median PF S of 3.4 months with chemotherapy alone v. 6.7 months with bevacizumab-containing therapy (p<0.001). Bevacizumab is the first anti-angiogenic agent which is associated with improved PFS when used in addition to standard chemotherapy regimens, both as first-line therapy and in the setting of recurrence. Our study was not randomized and included a small number of patients.
Bevacizumab therapy for EOC is an example of target-based therapy. Two major randomized trials have shown modest benefit in PF S (about 4 months) but not in overall survival with increased but manageable toxicity.[6],[7] Whether a lower dose of bevacizumab (5 mg/kg) will be equally effective or giving bevacizumab after completion of standard adjuvant chemotherapy will provide the same benefit remains to be answered.
Conclusion
Patients treated with bevacizumab had more frequent and higher grade of side-effects such as hypertension and minor bleeding complications. Our study suggests that addition of bevacizumab for treatment of EOC increases the toxicity of chemotherapy.
Acknowledgement
We acknowledge the support of Roche Pharma for providing bevacizumab for the study.
Conflicts of interest: None declared
| 1. | Agarwal S, Malhotra KP, Sinha S, Rajaram S. Profile of gynecologic malignancies reported at a tertiary care center in India over the past decade: Comparative evaluation with international data. Indian J Cancer 2012;49:298-302. [Google Scholar] |
| 2. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [Google Scholar] |
| 3. | Belotti D, Calcagno C, Garofalo A. Vascular endothelial growth factor stimulates organ-specific host matrix metalloproteinase-expression and ovarian cancer invasion. Mol Cancer Res 2008;6:525-34. [Google Scholar] |
| 4. | Yu L, Deng L, Li J, Zhang Y, Hu L. The prognostic value of vascular endothelial growth factor in ovarian cancer: A systematic review and meta-analysis. Gynecol Oncol 2013;128:391-6. [Google Scholar] |
| 5. | Micha JP, Goldstein BH, Rettenmaier MA, Genesen M, Graham C, Bader K, et al. A phase II study of outpatient first-line paclitaxel, carboplatin, and bevacizumab for advanced-stage epithelial ovarian, peritoneal, and fallopian tube cancer. Int J Gynecol Cancer 2007;17:771-6. [Google Scholar] |
| 6. | Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Gynecologic Oncologic Group Phase III trial of bevacizumab (BEV) in the primary treatment of advanced epithelial ovarian cancer (EOC), primary peritoneal cancer (PPC), and fallopian tube cancer (FTC): A Gynaecologic Oncology Group study. N Engl J Med 2011;365:2473-83. [Google Scholar] |
| 7. | Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Lauraine EP, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 2011 ;365:2484-96. [Google Scholar] |
| 8. | Burger RA. Experience with bevacizumab in the management of epithelial ovarian cancer. J Clin Oncol 2007;25:2902-8. [Google Scholar] |
| 9. | Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: A randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol 2012;30:2039-45. [Google Scholar] |
| 10. | Liu Y, Ren Z, Xu S, Bai H, Ma N, Wang F. Low-dose-intensity bevacizumab with weekly irinotecan for platinum- and taxanes-resistant epithelial ovarian cancer. Cancer Chemother Pharmacol 2015;75:645-51. [Google Scholar] |
| 11. | Lauraine EP, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol 2014;32:1302-8. [Google Scholar] |
| 12. | Akers SN, Riebandt G, Miller A, Groman A, Odunsi K, Lele S. Bevacizumab for the treatment of recurrent ovarian cancer: A retrospective cohort study. Eur J Gynaecol Oncol 2013;34:113-19. [Google Scholar] |
| 13. | Therasse P, Arbuck SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer. National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. [Google Scholar] |
| 14. | Avastin full prescribing information: Warnings. Available at www.gene.com/gene/products/information/oncology/avastin/insert.jsp#warnings (accessed on 10 Jan 2017). [Google Scholar] |
| 15. | Gordon MS, Cunningham D. Managing patients treated with bevacizumab combination therapy. Oncology 2005;69 (Suppl 3):25-33. [Google Scholar] |
Fulltext Views
1,683
PDF downloads
10,696




